About me
I am a clinical scientist interested in neuroimmunology, data analysis and single cell transcriptomics. I work as a resident physician in the Department of Neurology of the University Hospital Münster and am part of the mzhlab.
- Neuroimmunology
- Data Science
- Single cell transcriptomics
Datasets
Uveitis
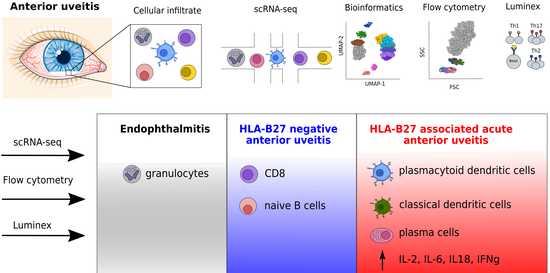
Single cell atlas of intraocular leukocytes in HLA-B27-positive and -negative uveitis
Neuro-COVID
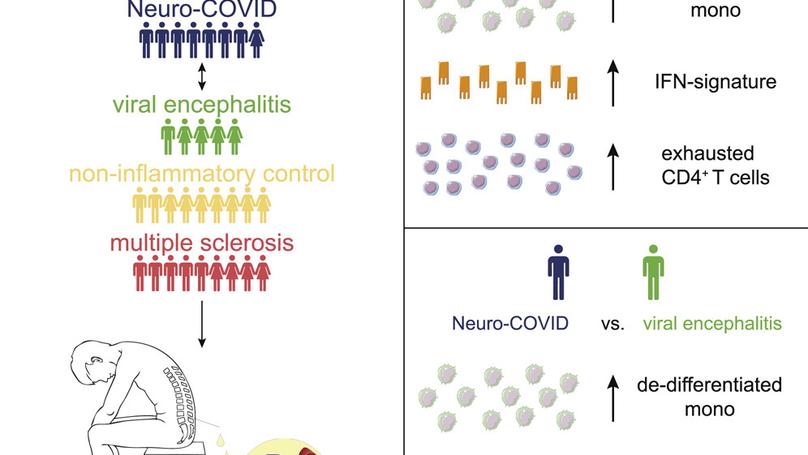
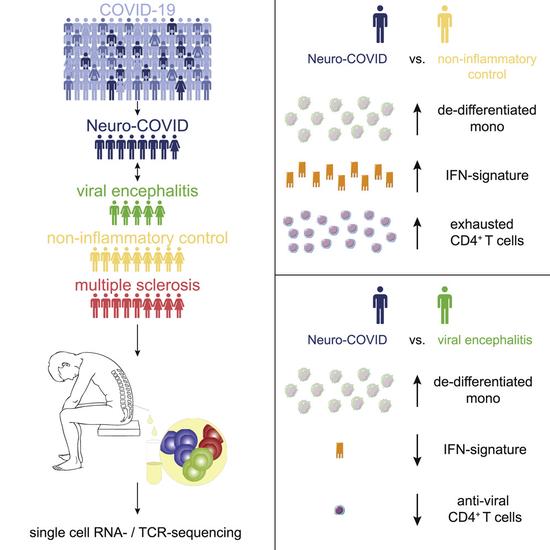
Single-cell atlas of cerebrospinal fluid in Neuro-COVID and controls
Murine peripheral nerve
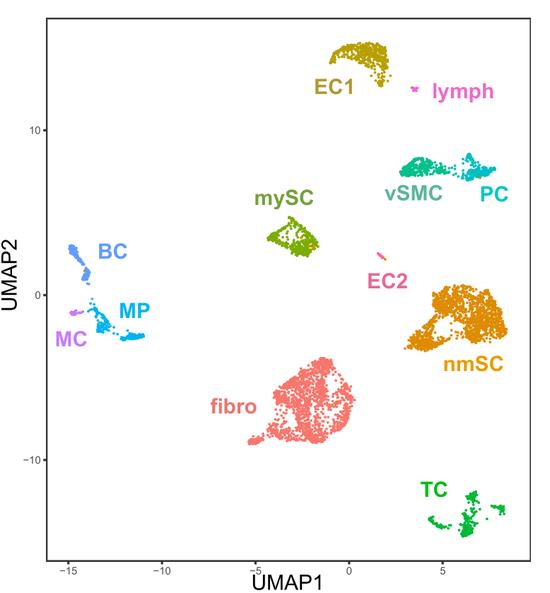
Redefining the heterogeneity of peripheral nerve cells in health and autoimmunity
Neurosarcoidosis
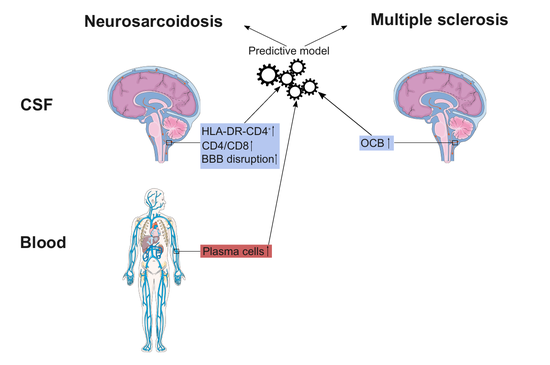
Differentiating neurosarcoidosis from multiple sclerosis using flow cytometry.
Recent Publications
Featured Publications
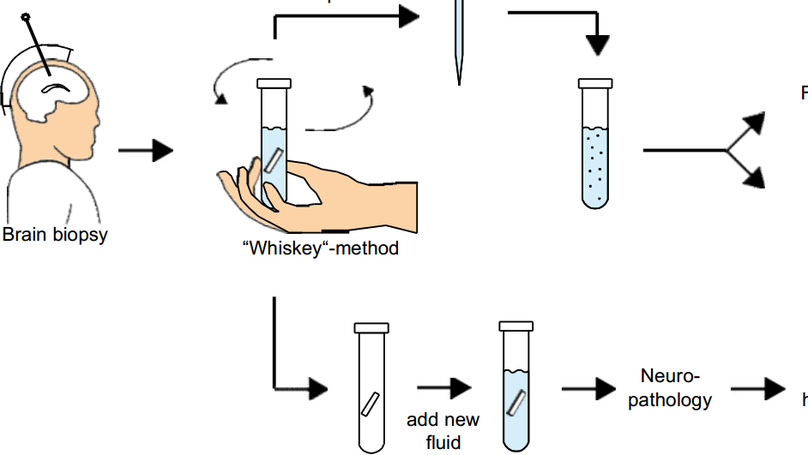
BACKGROUND: Primary central nervous system lymphoma (PCNSL) is a rare lymphoma of the central nervous system, usually of diffuse large B cell phenotype. Stereotactic biopsy followed by histopathology is the diagnostic standard. However, limited material is available from CNS biopsies, thus impeding an in-depth characterization of PCNSL. METHODS: We performed flow cytometry, single-cell RNA sequencing, and B cell receptor sequencing of PCNSL cells released from biopsy material, blood, and cerebrospinal fluid (CSF), and spatial transcriptomics of biopsy samples. RESULTS: PCNSL-released cells were predominantly activated CD19 + CD20 + CD38 + CD27 + B cells. In single-cell RNA sequencing, PCNSL cells were transcriptionally heterogeneous, forming multiple malignant B cell clusters. Hyperexpanded B cell clones were shared between biopsy- and CSF- but not blood-derived cells. T cells in the tumor microenvironment upregulated immune checkpoint molecules, thereby recognizing immune evasion signals from PCNSL cells. Spatial transcriptomics revealed heterogeneous spatial organization of malignant B cell clusters, mirroring their transcriptional heterogeneity across patients, and pronounced expression of T cell exhaustion markers, co-localizing with a highly malignant B cell cluster. CONCLUSIONS: Malignant B cells in PCNSL show transcriptional and spatial intratumor heterogeneity. T cell exhaustion is frequent in the PCNSL microenvironment, co-localizes with malignant cells, and highlights the potential of personalized treatments.
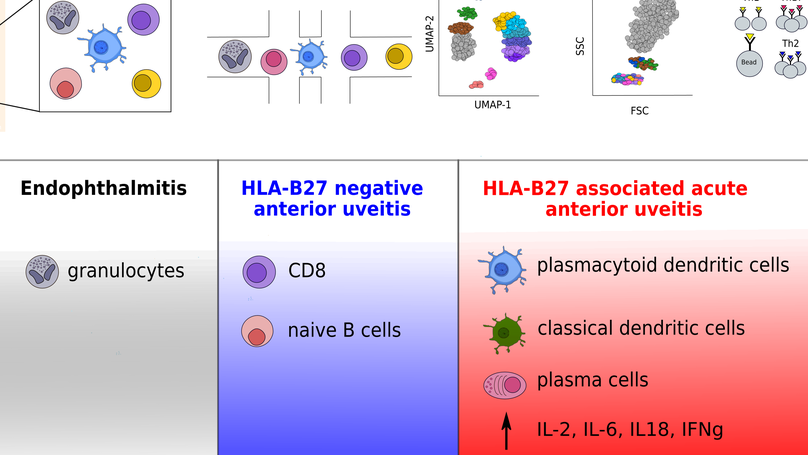
Uveitis describes a heterogeneous group of inflammatory eye diseases characterized by infiltration of leukocytes into the uveal tissues. Uveitis associated with the HLA haplotype B27 (HLA-B27) is a common subtype of uveitis and a prototypical ocular immune-mediated disease. Local immune mechanisms driving human uveitis are poorly characterized mainly due to the limited available biomaterial and subsequent technical limitations. Here, we provide the first high-resolution characterization of intraocular leukocytes in HLA-B27-positive (n = 4) and -negative (n = 2) anterior uveitis and an infectious endophthalmitis control (n = 1) by combining single-cell RNA-sequencing with flow cytometry and protein analysis. Ocular cell infiltrates consisted primarily of lymphocytes in both subtypes of uveitis and of myeloid cells in infectious endophthalmitis. HLA-B27-positive uveitis exclusively featured a plasmacytoid and classical dendritic cell (cDC) infiltrate. Moreover, cDCs were central in predicted local cell-cell communication. This suggests a unique pattern of ocular leukocyte infiltration in HLA-B27-positive uveitis with relevance to DCs.
We performed basic CSF analysis and multi-dimensional flow cytometry of CSF and blood cells from 59 patients with primary psychotic disorders in comparison to inflammatory and non-inflammatory controls. We found an expansion of monocytes in the blood and CSF of psychosis patients. A machine learning model incorporating blood and CSF parameters differentated psychosis from non-inflammatory controls better than individual paramaters.
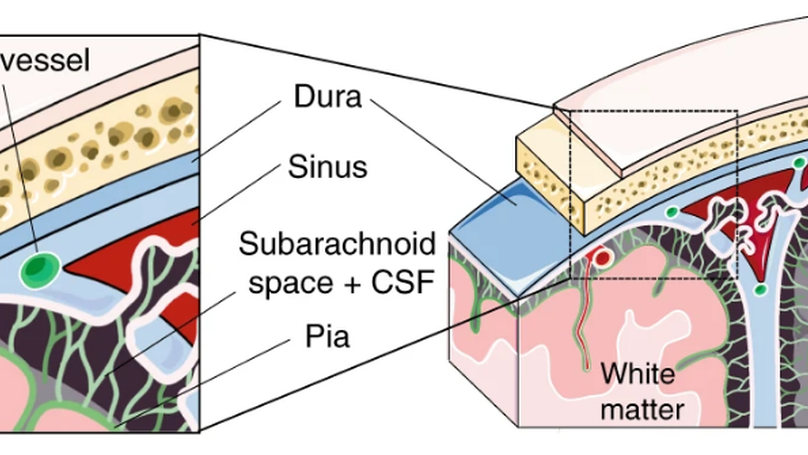
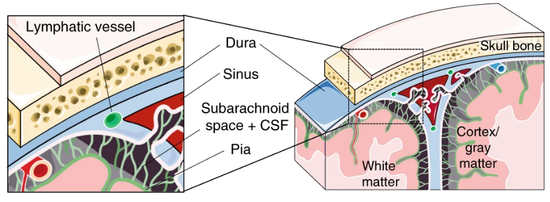
Based on single-cell transcriptomics, we here identify a highly location-specific composition and expression profile of tissue-resident leukocytes in CNS border compartments featuring B cells and their progenitors in the dura as an unexpected site of B cell residence.